 Wiki90
Wiki90
Wiki90: 90s Style Encyclopedia on the Web

|

|

|

|




Ammonium oxalate
In this article we are going to address the issue of Ammonium oxalate, which is of utmost importance in the current context. Ammonium oxalate can refer to a wide range of topics, from the importance of education in today's society, to the life and work of a relevant character in history. Whatever its nature, Ammonium oxalate is a topic that arouses the interest of many people, as it has a significant impact on our lives. Throughout this article we will explore different aspects of Ammonium oxalate, analyzing its relevance and the role it plays in different contexts. In addition, we will examine different perspectives and opinions on the matter, with the aim of enriching our knowledge on this very relevant topic.
| |
| Names | |
|---|---|
| IUPAC name
Ammonium oxalate
| |
| Systematic IUPAC name
Ammonium ethanedioate | |
| Other names
Diammonium oxalate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.012.912 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
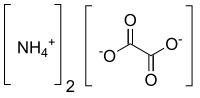
| [NH4]2C2O4 | |
| Molar mass | 124.096 g·mol−1 |
| Appearance | Colorless or white crystalline solid |
| Density | 1.5 g/cm3 |
| Melting point | 70 C (158 F, 343.15 K) |
| 5.20 g/(100 ml) (25 °C) | |
| Hazards | |
| GHS labelling: | |
| H302, H312, H319 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ammonium oxalate is a chemical compound with the chemical formula [NH4]2C2O4. Its formula is often written as (NH4)2C2O4 or (COONH4)2. It is an ammonium salt of oxalic acid. It consists of ammonium cations ([NH4]+) and oxalate anions (C2O2−4). The structure of ammonium oxalate is ([NH4]+)2[C2O4]2−. Ammonium oxalate sometimes comes as a monohydrate ([NH4]2C2O4·H2O). It is a colorless or white salt under standard conditions and is odorless and non-volatile. It occurs in many plants and vegetables.
Vertebrate
It is produced in the body of vertebrates by metabolism of glyoxylic acid or ascorbic acid. It is not metabolized but excreted in the urine. It is a constituent of some types of kidney stone. It is also found in guano.
Mineralogy
Oxammite is a natural mineral form of ammonium oxalate. This mineral is extremely rare. It is an organic mineral derived from guano.
Chemistry
Ammonium oxalate is used as an analytical reagent and general reducing agent. It and other oxalates are used as anticoagulants, to preserve blood outside the body.[citation needed]
Earth sciences
Acid ammonium oxalate (ammonium oxalate acidified to pH 3 with oxalic acid) is commonly employed in soil chemical analysis to extract iron and aluminium from poorly-crystalline minerals (such as ferrihydrite), iron(II)-bearing minerals (such as magnetite) and organic matter.[page needed]
References
- ^ a b John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 4–41. ISBN 978-1138561632.
- ^ a b National Center for Biotechnology Information. PubChem Compound Database; CID 14213 (accessed 15 November 2016).
- ^ The International Pharmacopoeia, p.1292, Volume 1, World Health Organization, 2006 ISBN 92-4-156301-X.
- ^ N G Coley, "The collateral sciences in the work of Golding Bird (1814–1854)", Medical History, iss.4, vol.13, October 1969, pp.372.
- ^ "Home". mindat.org.
- ^ Rayment, George; Lyons, David (2011). Soil Chemical Methods - Australasia. CSIRO Publishing. ISBN 9780643101364.
 Ammoniumoxalat
Ammoniumoxalat Ammoniumoksalaatti
Ammoniumoksalaatti Oxalate d'ammonium
Oxalate d'ammonium Ossalato di diammonio
Ossalato di diammonio Ammoniumoxalaat
Ammoniumoxalaat Szczawian amonu
Szczawian amonu Oxalato de amônio
Oxalato de amônio Amonyum okzalat
Amonyum okzalat

